Can viruses be friendly? We see viruses as unbeatable killers. Do they act differently? Phage Therapy (PT) or Bacteriophage Therapy is the answer. Phages are beneficial viruses that kill harmful bacteria. These micro warriors ubiquitous in nature thrive in harmful bacteria and are harmless to humans, animals, and plants.
Antibiotic overuse has led to growing antimicrobial resistance (AMRs), which if not checked can have severe health and economicimpact. Nearly 700,000 deaths happen every year from antimicrobial resistance (WHO Reports).
According to research commissioned by the UK government, antimicrobial infections could cause 10 million deaths per year globally by 2050, with an economic impact equivalent to the 2008 financial crisis. This potential threat has created a renewed interest in revisiting Phage Therapy.
Phage Therapy has opened new frontiers to antibiotic treatments. How can it help us? This guide explores the fascinating domain of Phage Therapy, its concept,history, mechanism of action, and future potential.
Guide to Phage Therapy – Concept, History, Application and Future Potential
-
Concept of Phage Therapy – A promising solution to antimicrobial resistance?
-
Understanding Bacteriophages and their role in Phage Therapy
-
The History of Phage Therapy
-
How Phage Therapy Works?
-
What conditions can be treated with Phage Therapy?
-
Future Prospects and Advancements in Phage Therapy
-
Pros and Cons of Phage Therapy
Concept of Phage Therapy – A promising solution to antimicrobial resistance?
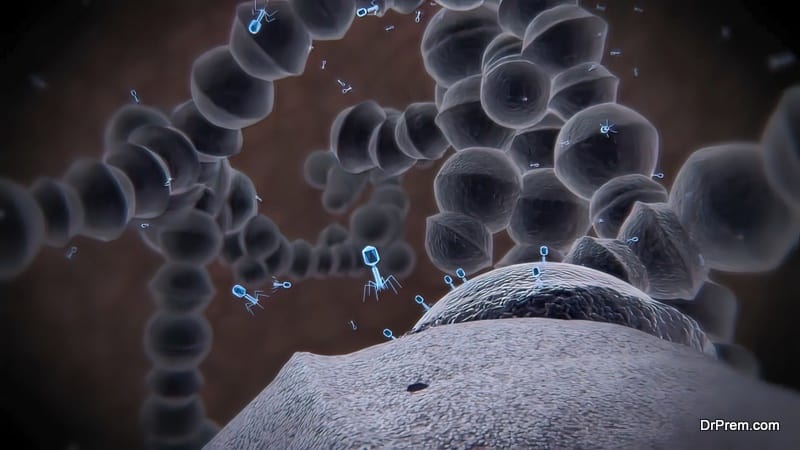
Phage Therapy involves naturally-occurring Phages (bacteriophages) infecting the bacteria by breaking down the bacterial plasma membrane. Bacteriophages are found in soil, water, and other places where other bacteria thrive. Biotechnological advances have further expanded the potential of phage therapy using engineered phages and improved lytic proteins.
The uniqueness of Phage Therapy lies in its host specificity, which means, one specific strain works against one specific strain of bacteria. Most phages kill a specific species of bacteria by lysing specific strains of that species. They don’t function as broad-spectrum antibiotics which kill beneficial bacteria along with the harmful clan. This limited host specificity is less harmful to our bodies as it does not disrupt the natural body flora and prevents the risk of future bacterial infections.
Just when the world is on the brink of losing the miracle power of antibiotics, Phage Therapy emerges as the smart biological bomb that can be used as a targeted weapon to kill harmful bacteria.
Understanding Bacteriophages and their role in Phage Therapy
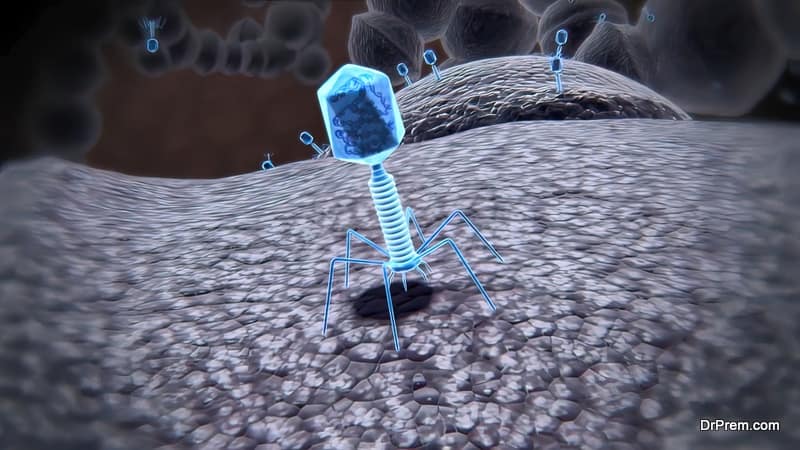
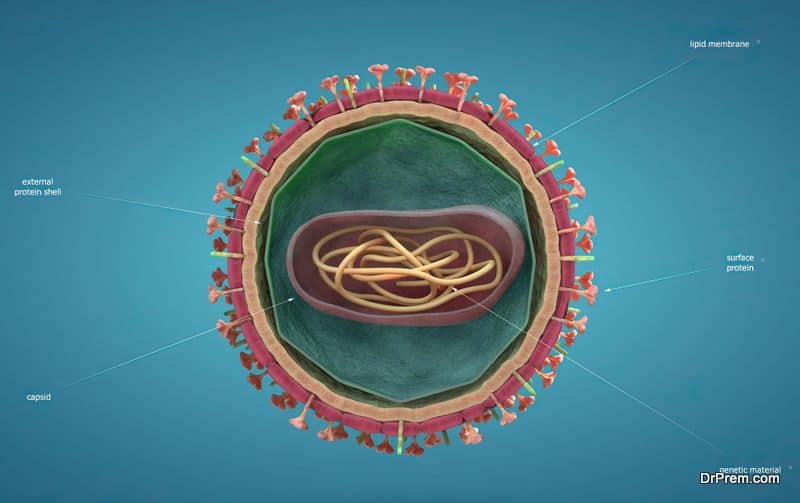
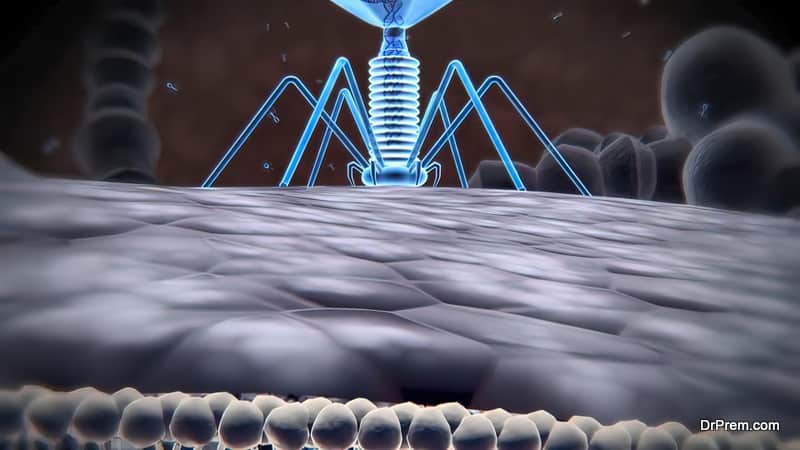
In Latin, bacteriophages mean “bacteria eaters”. Just like other viruses, Phages or Bacteriophages are microorganisms with nucleic acid (DNA or RNA) at the core covered by a protein capsid. Phages are smaller than bacteria in size and present three different structural forms – an icosahedral head (20-sided) and a tail, only an icosahedral head without the tail, or a filament.
Phages are ubiquitous in our surroundings and highly diverse in size, genetic organization, and morphology. Their unique protein-protein interactions make them useful in human health applications, food science, and safety. Phages can also be used in drug delivery and vaccine development. They can also play a great role in designing stable sensors to detect bacteria or help in specific diagnostics.
The History of Phage Therapy
D’Herelle was quick to understand the inherent potential of these natural bacterial antagonists to treat bacterial infections. The breakthrough came in 1919 when he successfully applied Phages to cure dysentery of four patients, thus heralding a unique journey of treating microbial infections.
D’Herelle was successful in restrictingoutbreaks of cholera in India and plague in Egypt with Phage Therapy. In 1923, two physicians from Baylor University’s College of Medicine also reported success in one Phage Therapy trial in the US. They also came to the conclusion that Phages hold enormous potential in fighting deadly infectious diseases.
Unfortunately, less-than-expected outcomes of clinical trials of Phage therapy slowed the enthusiasm surrounding it possibly due to overhyped clinical trial outcomesthat were not supposed to be. Although, this treatment was commercially launched in the US, Western Europe, and the early Soviet Union period, very few had D’Herelle’s level of expertise to drive the desired treatment outcome.
Experts failed to understand Phage biology. Treatment outcomes were not convincing enough which led to dwindling acceptance of this treatment. In 1932, a warning from an American Health Officer drove the final nail to the coffin. Poor clinical application and rapid commercial exploitation without sound learning and application led to the rejection of this promising treatment.
The widespread availability of cheap antibiotics promising to treat any infection further subsided the need to use Phage Therapy. Antibiotic infallibility became so granted that this therapy got buried under its popularity. The West discarded it outrightly considering it an unnecessary effort when the problem is already solved. Few could foresee the impending health threat in the form of antimicrobial resistance that would force a resurgence in Phage Therapy.
However, the therapy did not die out, courtesy George Eliava Institute of Bacteriophage, Microbiology, and Virology. Opened in 1923 in Tbilisi, the capital city of Georgia (a post-Soviet European country), this institute has been putting relentless efforts to make Phage Therapy more effective to combat infections without antibiotics. The founder Professor George Eliava learned about Phage Therapy when he met Felix d’Herelle on his visit to the Pasteur Institute in Paris.
Encouraged by the potential of Phage Therapy, he started working on it in his institute in Tbilisi. He invited d’Herelle to visit his lab in Georgia and work with him. D’Herelle, somewhat influenced by the communist philosophy, visited Tbilisi twice in 1933-34. In 1934, Joseph Stalin also invited D’Herelle to work in the institute. He dedicated his book The Bacteriophage and the Phenomenon of Cure’ to Stalin which was published in 1935.
However, the camaraderie between the two scientists did not last long. In 1937, when d’Herelle was about to settle in Georgia, Prof Eliava was executed after being labeled as the enemy of the people. Disheartened, D’Herelle went back to France. He was never allowed to return to Georgia and his book was also banned from distribution.
Despite such unfortunate happenings, the institute carried out bacteriophage research. In 1938, the Institute of Bacteriophage Research and the Institute of Microbiology and Epidemiology merged to form the Institute of Microbiology, Epidemiology, and Bacteriophage. In 1988, the institute was rearranged and the scientific portion was renamed the George Eliava Research Institute of Bacteriophage.
The institute as a general Soviet institute worked on developing bacteriophage drugs. Patients from all over the Soviet Union visited this institute to treat a range of serious infectious diseases. Phage Therapy became a routine treatment in all Soviet clinics and hospitals. Phage products were sold in the form of drops, ointments, pills, and washes, which are still available in Eastern European countries at a low price.
This Tbilisi institute faced massive destruction after the Republic of Georgia refused to join the Russian Federation. All the research work on bacteriophage was destroyed. Thousands of preserved bacteriophage samples suffered irreversible damage due to severe power outages during the 1991 civil war in Georgia. The Russians could manage to transfer some of the essential equipment to their country leaving this institute devastated and on the brink of closure.
However, it saw the lights of revival in 1997 based on a BBC broadcast that highlightedits remarkable work. This caught the attention of scientists, doctors, and enthusiastic entrepreneurs from around the world whose initiatives put the institute up and running.
It is clear that bacteriophages hold specific advantages over conventional chemotherapy. This is because Phages are extremely precise and targeted while attacking bacteria. This factor often collided rather than aiding in effective treatment during d’Herelle’s time. This would not be a problem today with advanced diagnostic technologies facilitating rapid selection of phages for specific infections.
How Phage Therapy Works?
It is a unique mechanism where Phages take over the functioning of bacterial cells and function as per their wish. The mechanism is almost the same as the normal hijacking system of viruses.
During infection, a phage attaches itself to the bacterial cell and inserts its genetic material into it. It is normally followed by either one of the lifecycles – the lytic phage (virulent) when it hijacks the bacterial cell to create numerous components of phage by lysing or destroying the bacterial cell membrane.
The other phage, the Lysogenic phage inserts its nucleic material into the chromosome of bacteria and starts replicating as a unit without destroying the bacterial cell. In some circumstances, the Lysogenic Phage can be made to follow the Lytic cycle.
Apart from this, there are other lifecycles also – pseudolysogeny and chronic infection. In pseudolygoney, the phage inserts its material into the bacterial cell but neither it lyses nor replicates. This lifecycle happens due to unfavorable growth conditions in the bacterial cell.
The phage survives in that condition waiting to replicate or lyse till the conditions become favorable for growth. In chronic infections, new phage components keep on proliferating for a long time without killing the host cell.
What makes Phages unique? It is that they replicate only in bacterial cells.
What conditions can be treated with Phage Therapy?
Can bacteria become resistant to Phages?
Phage-resistant variations lack these receptors and are often less pathogenic than the non-resistant ones. The development of phage resistance can be thwarted beforehand by using phage cocktails or in combination with other antibiotics.
Future Prospects and Advancements in Phage Therapy
Antibiotics have revolutionized medical treatment since its advent in 1928 but few could foresee its reduced efficacy after several decades. Phage Therapy was put on the back burner as we got a better weapon to kill bacteria.
The world did not invest enough to conclude the efficacy of Phage Therapy. Despite the looming skepticism about the efficacy of Phage Therapy, growing evidence and successful case studies signal its successful clinical applications in the near future.
Documented evidence of successful Phage therapy in Eastern Europe might not be accepted by the standard Western protocols but it cannot be discounted either.
Here are a few case studies:
- A blind study with more than 30,000 children by the health authorities of Tbilisi, Georgiain 1963 showed a significantly lesser incidence of diarrhea among those who received prophylactic Phage pill (1.8 cases per 1000) than the placebo group (6.7 cases per 1000).
- A 1983 study with several hundred patients with suppurative bacterial infections most of which are drug-resistant showed a 92.4% overall success rate with Phage Therapy.
- A recent 2009 double-blind Phase II clinical study proved the safety and efficacy of phage therapy in treating drug-resistant ear infections. Out of 12 patients receiving Phage Therapy, only 3 were cured and no adverse effects were reported for the remaining patients.
- Another 2009 randomized, double-blind clinical trial for treating venous leg ulcers (VLU) with 42 patients found no significant improvement in conditions than in the control groups. No adverse incident was reported either. A possible lag is that the Phages were not tested for their effectivity against the specific bacteria causing VLU.
- The largest clinical trial in Europe in 2013 with 27 patients with burn wounds showed a decrease in bacterial load with Phage Therapy but the progress was slower than that of the control group. No adverse condition with Phage Therapy was reported. This limited efficacy of the Phage cocktail was attributed to the significant drop in the Phage titer where the patients received very lower concentrations of Phages to fight the bacteria.
- The startling success of Phage Therapy was reported by a 68-year-old patient in 2019 suffering from necrotizing pancreatitis where multiple rounds of antibiotic treatments failed. Intravenous Phage administration rapidly improved the patient’s condition by destroying the infection.
Phage Therapy is yet to gain unequivocal evidence of success. The main reason for the partial success of Phage Therapy is the lack of deep understanding of Phage specificity, that is finding the right strain or cocktail to fight a specific bacterial strain. Despite this, the number of successful case studies to treat life-threatening infections is increasing.
Future Prospects
- Targeted Phage selection harnessing genomics and bioinformatics. Target-specificity is key to the successful application of Phage Therapy. It should be compatible with the genomic constitution of an individual and the bacteria causing their suffering.
- Phages can be re-engineered for a targeted attack on bacterial infections with innovative Phage cocktails.
- Better prospects of personalized treatments by creating personalized Phage cocktails.
- Effective combination therapies with antibiotics and Phages.
Pros and Cons of Phage Therapy
Pros
- The biggest benefit is its ability to treat antimicrobial-resistant bacteria.
- Phage Therapy can be combined with other antibiotics and drugs.
- Phages facilitate “Auto-dosing” as they can replicate faster while killing bacterial cells specifically targeting the host location. Though there is a limitation as it depends on relatively high bacterial loads.
- Their replicative nature may need a much lower dose of drugs. A single dose could be enough to treat a specific infection.
- They are harmless as discovered so far and do not kill beneficial bacteria like other antibiotics.
- They can be found easily.
- Relatively low cost of treatment
- Higher potential for treatment versatility
- Fewer chances of developing Phage-resistance
Cons
Scientific and logistical challenges are big barriers to the advancements of Phage Therapy.
- The efficacy of Phage Therapy depends on several factors. An improved understanding of pharmacokinetics is needed to enhance the therapeutic value of Phage Therapy.
- There are several hurdles regarding manufacturing, preserving, and distributing Phage products that need a highly-coordinated advanced infrastructure.
- Phages might trigger an immune response.
- Phages may not be effective in treating all types of bacterial infections.
- Advanced interdisciplinary thinking and treatment approaches from biotechnology professionals and healthcare experts are needed to make Phage Therapy applicable on a large scale.
- Unprecedented regulatory hurdles.Stringent protocols may render Phage Therapy prohibitively expensive.
Can Phage Therapy be the holy grail of treating drug-resistant infections? The jury is still out. Expectations are high that a better understanding of Phages, their target specificity, and engineered solutions can make Phages magical antibacterial agents reducing the need for antibiotics. Ongoing research and studies signify that this therapy has enough potential for universal acceptance in Western medicine. Are we entering an antibiotic-free world?