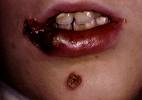
The U.S. Food and Drug Administration has approved Altabax, a new antibacterial for treatment of impetigo.
The drug is the first in a new class of antibacterial agents called pleuromutilins, which inhibit protein synthesis in bacteria.
The introduction of Altabax comes at a time when antibiotic resistance is at an increasingly high level, said Dr. Stan Block, president of Kentucky Pediatric and Adult Research Inc. Altabax provides clinicians with a convenient new means to effectively fight the bacteria that cause <a href="impetigo through an effect that is different from other antibiotics.
Dr. Stan Block said bacteria were less likely to develop resistance to Altabax than to drugs currently in use.
The drug has been approved for use in children 9 months or older. It is used twice a day for a five-day period. Older treatments have tended to need to be applied for more than twice as long, and have also required more treatments per day.
Impetigo is a skin infection caused by the common staph aureus and strep pyogenes bacteria. It is especially common in children, because their immune systems are not fully developed. It spreads easily through physical contact with someone who has impetigo, or from sharing clothes, bedding, towels, or other objects.
Altabax is manufactured by GlaxoSmithKline, Research Triangle Park, NC 27709.
Via : WebMD.com