Pediatric cancer treatments have advanced a lot since the last ten years with survival rates jumping to 90% from 60% in the mid-1970s. When standard cancer treatments do not work or there is a relapse, CART cell immunotherapies for children are highly promising. These treatments utilize the body’s inherent power of self-protection to attack cancer cells.
This latest treatment for children with leukemia has generated lots of excitement. According to Stanford Medicine, about 10,000 children aged 14 and less are diagnosed with cancer every year with Acute Lymphoblastic Lymphoma (ALL), accounting for 1/3rd of all the pediatric cancer cases.
ALL is one of the most treatable forms of pediatric leukemia, with more than 90% of children responding well to the conventional treatments achieving remission. But the problem is with children either being non-respondent to conventional treatments or facing a relapse after a few years of remission.
FDA approves the first CAR T cell pediatric immunotherapy:

Sensing the success in CAR T cell immunotherapies for children, researchers at NCI and other organizations have been seriously engaged in expansion of these therapies for other types of cancer.
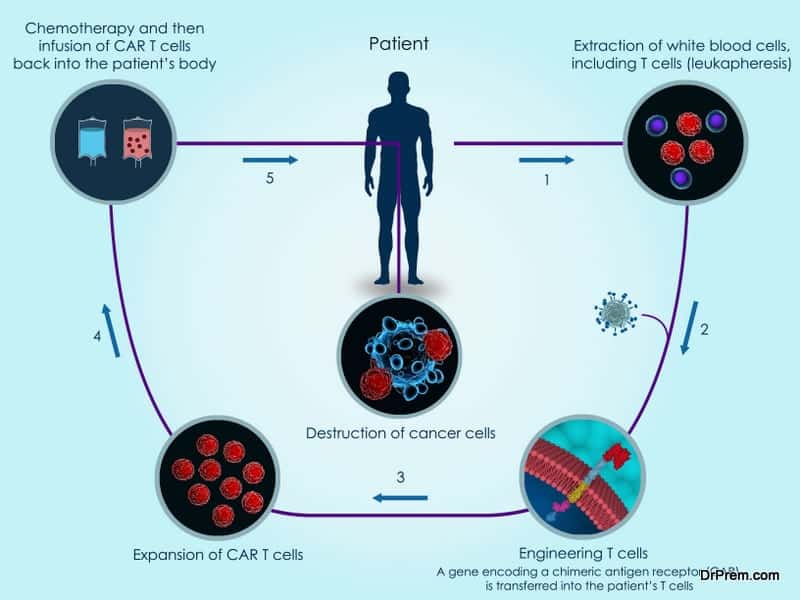
CAR T cell immunotherapies for children –teaching the body to recognize cancer cells:
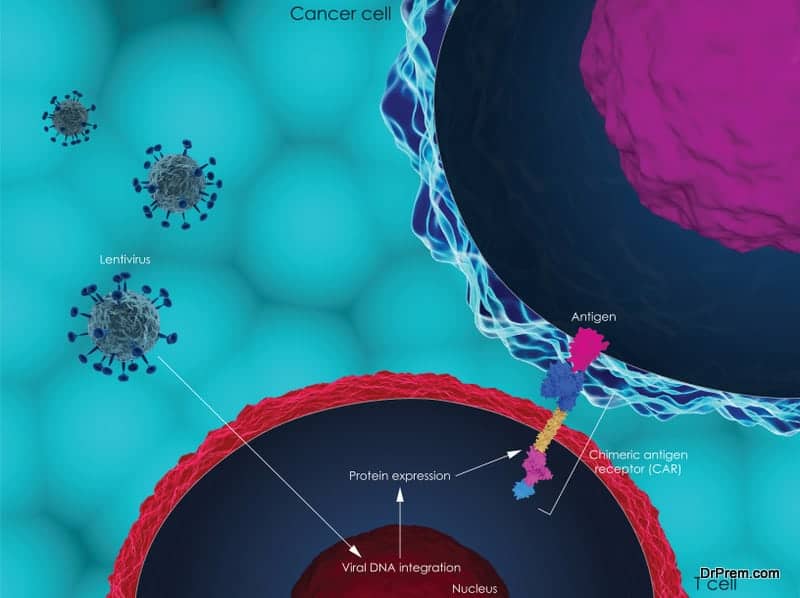
T cells present in the white blood cells are instrumental in building the body’s immune system. Our immune system is constantly patrolling our body, killing pathogens without us even knowing about it. But it may always not be able to recognize the cancer cells as an invader.
CAR T cell immunotherapies for children imply teaching the body’s immunity system to recognize cancer cells as an “enemy”. The engineered T cells look for leukemia cells with the CD 19 protein and kill them.
How is CAR T cell therapy leukemia performed?
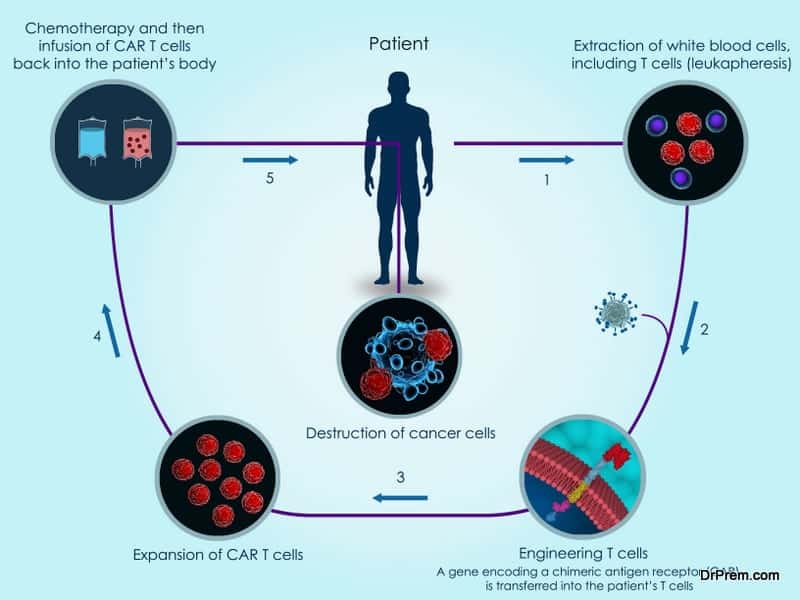
- The first step involves in collecting the blood from the child in a hospital. The T cells are removed from the blood and the remaining blood is again transfused in the patient’s body.
- The T cells are then shipped to a cell-processing lab for genetic modification. In the lab, the T cells are engineered by arming them with new receptors known as Chimeric Antigen Receptors or CARs. Armed with these receptors, the CAR T cells can identify and bind with the cells having CD 19 antigen.
- The CAR T cells are multiplied in the lab to enhance their capacity to attack more cancer cells. After the desired level of multiplication, the cells are frozen and brought back to the hospital or clinic for reinfusion.
- The patient has to go through the lymphodepleting chemotherapy before the transfusion of engineered T-cells. Post-transfusion, the patient is kept under strict surveillance.
Once the modified cells are in the patient’s body, it is likely that they would create more CAR T cells to make the treatment effective for a long time.
Potential risks and managing the side-effects in CAR T cell immunotherapies for children:
To counter this action, FDA has also approved the arthritis drug Actemra along with Kymriah. In CAR T cell therapy clinical trials, one or two doses of Actemra reduced the CRS symptoms completely within two weeks. In many patients, the symptoms could be checked with steroids.
Children post-CAR T treatment require a monthly infusion of immunoglobin to prevent further infections in the future. This is because the engineered cells kill normal B cells with CD19 along with the cancerous ones.
Cerebral Edema is another potentially life-threatening side effect of CAR T therapy. This has been observed in larger clinical trials on patients with advanced leukemia, forcing one company to halt the trial. Thankfully, this problem seemed to be negligible as other leading trials did not report such side effect.
New CAR T cell immunotherapies for children with relapsing cancer:
In about 50% of the relapsed leukemia cases, it has been observed that the T cells could not recognize cancer cells. It means that the cells did not have the CD 19 protein that normally T cells bind with.
In a new approach to CAR T cell immunotherapies for children, the T-cells are armed with receptors CD19 and CD22, another protein found in leukemia cells. In the first clinical trial of CD22 targeted CAR T therapy, most patients saw complete remission in spite of the relapse after a complete CD19 targeted CAR T therapy.
However, relapses after CD22-targeted immunotherapy are not uncommon either. It is also important to target CD123, another protein common in leukemia cells.Therefore, the ultimate approach is to develop multi-antigen targeted CAR T therapy.
Early phase clinical trials of T cell engineering targeting CD19 and CD22 are on. Some researchers are also testing CAR T targeting CD19 and CD123 proteins. In studies with animals, it has been found that dual targeting may prevent antigen escape.
Hindrances to CAR T cell immunotherapy:

Moreover, the entire procedure is quite consuming. It takes roughly2-4 weeks in getting the patient’s cells engineered in the lab. Within that time, the physicians have to battle in keeping an acutely ill patient alive.
Research is on to genetically engineer the cells within the patient’s body, eliminating the need to transfer their cells to the lab for the purpose. This may make the treatment safer, faster, and cheaper as there would be no need for customization for each patient.
The FDA approval in immunotherapy indeed has made a difference. Children with relapsed or refractory ALL who would have died few years ago now stand a fair chance of survival. Researchers are also looking for improved immunotherapies with better cancer cell recognition capabilities. The ultimate goal is to save everychild with relapsed ALL from the jaws of death.